Osteoporosis Risk Assessment Tool
Assess Your Osteoporosis Risk Factors
This tool evaluates your risk based on immune-related and conventional factors that influence bone health.
Your Osteoporosis Risk Assessment
Based on your inputs, your risk level is:
Recommended Actions:
Quick Summary
- Bone health is tightly linked to immune activity through a process called osteoimmunology.
- Inflammatory cytokines drive the cells that break down bone, increasing fracture risk.
- Hormones like estrogen and nutrients such as vitaminD regulate both immunity and bone remodeling.
- Targeting the RANKL pathway can treat bone loss while also modulating immune responses.
- Practical steps - anti‑inflammatory diet, regular weight‑bearing exercise, and screening for chronic inflammation - help protect bone density.
What Is Osteoporosis is a chronic condition characterized by reduced bone mass and structural deterioration, making bones fragile and prone to fractures?
When bone becomes porous, everyday activities like carrying groceries or climbing stairs can cause a break. The condition affects roughly 200 million people worldwide, with women over 60 carrying the highest burden. Diagnosis usually relies on a bone mineral density (BMD) scan, where a T‑score of -2.5 or lower signals osteoporosis. While genetics, age, and lifestyle are well‑known drivers, researchers have uncovered a hidden partner: the immune system.
Why the Immune System is the body’s defense network of cells, tissues, and signaling molecules that protect against pathogens and maintain internal balance matters for bone
The immune system constantly surveys the body, releasing messenger proteins called cytokines. Some cytokines calm inflammation; others stoke it. Bone tissue lives in that same environment, so chronic immune activation can tilt the balance toward bone loss. Think of bone as a construction site: immune signals act like foremen who either speed up demolition or boost rebuilding.
Bone Remodeling: The Cellular Dance
Bone never stays static. Two cell types choreograph its turnover:
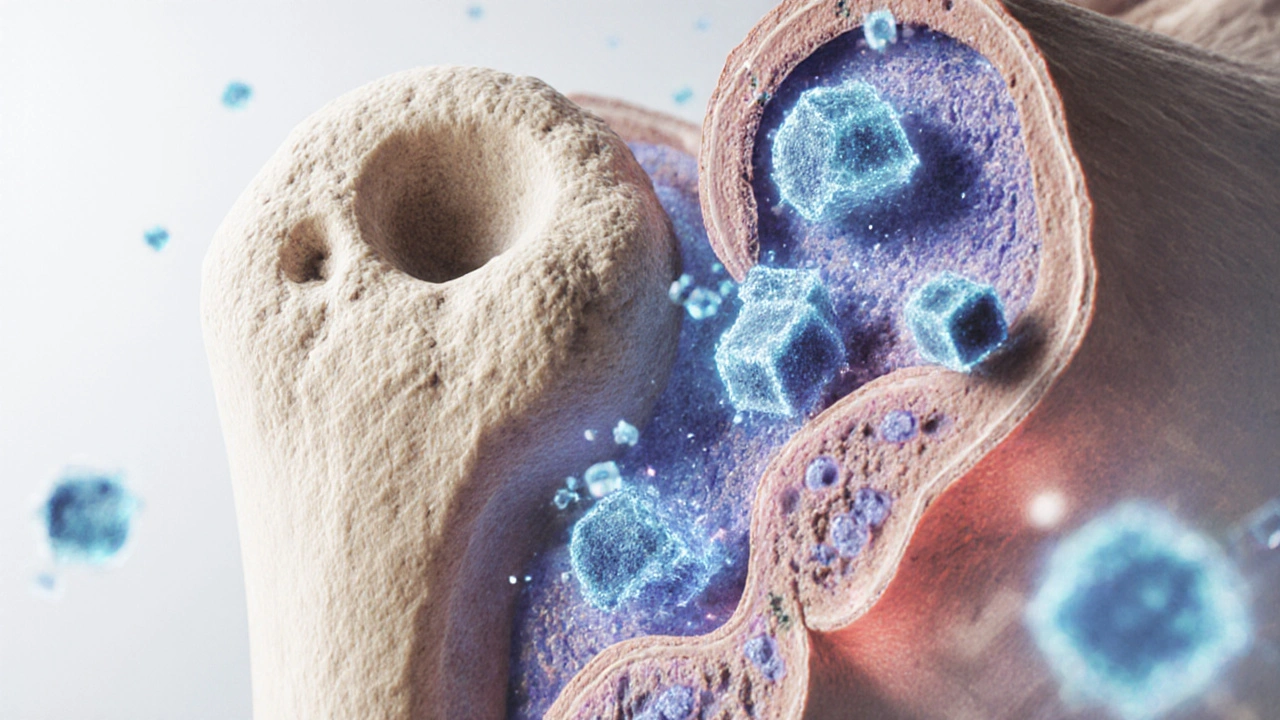
- Osteoclast is a large, multinucleated cell that resorbs (breaks down) bone tissue. It creates tiny pits called resorption lacunae.
- Osteoblast is a bone‑forming cell that fills those pits with new mineralized matrix. It later matures into an osteocyte, which monitors strain.
In a healthy adult, osteoclast activity roughly equals osteoblast activity - a steady state. When the scales tip, bone density shifts.
Osteoimmunology: The Bridge Between Bones and Immunity
The term osteoimmunology describes the crosstalk between Bone Remodeling is the continuous process of bone resorption and formation that maintains skeletal strength and immune signaling. Central to this dialogue are two players: Cytokine is a small protein released by immune cells that influences the behavior of other cells and the receptor‑ligand pair RANKL is a key molecule that binds to RANK on osteoclast precursors, prompting them to mature and become bone‑resorbing cells.
When pro‑inflammatory cytokines such as TNF‑α, IL‑1β, and IL‑6 rise, they boost RANKL expression on osteoblasts and immune cells. The result? More osteoclasts, more bone erosion, and higher fracture risk.
Key Molecular Pathways
Below are the most influential routes that link immune activity to bone loss:
- RANK/RANKL/OPG axis: RANKL binds RANK on osteoclast precursors; osteoprotegerin (OPG) acts as a decoy. Inflammation raises RANKL and lowers OPG, accelerating bone resorption.
- NF‑κB signaling: Many cytokines trigger NF‑κB, a transcription factor that drives osteoclast gene programs.
- JAK/STAT pathway: Cytokines such as IL‑6 activate STAT3, which also up‑regulates RANKL.
Targeted drugs like denosumab (a monoclonal antibody against RANKL) illustrate how dampening this pathway can simultaneously treat osteoporosis and certain inflammatory diseases.
Hormonal and Nutritional Modulators
Two non‑immune factors have double duty - they affect both immunity and bone:
- Estrogen is a sex hormone that suppresses bone resorption and modulates cytokine production. Post‑menopausal estrogen loss spikes IL‑1 and TNF‑α, explaining the sharp rise in osteoporosis among women.
- Vitamin D is a fat‑soluble vitamin that promotes calcium absorption and also regulates immune cell function. Deficiency correlates with higher CRP (a marker of inflammation) and lower BMD.
Ensuring adequate vitaminD (800‑1000IU daily for most adults) and maintaining healthy estrogen levels (through lifestyle or, where appropriate, hormone therapy) can blunt the inflammatory cascade that harms bone.
Lifestyle Triggers that Tie Inflammation to Bone Loss
Modern habits often fuel low‑grade inflammation, a silent driver of osteoporosis:
| Category | Specific Factor | How It Affects Bone |
|---|---|---|
| Immune‑related | Chronic periodontal disease | Elevates IL‑6 and RANKL, increasing osteoclast activity. |
| Immune‑related | Rheumatoid arthritis | Autoimmune cytokine surge directly accelerates bone erosion. |
| Conventional | Age > 65 | Reduced osteoblast function, higher fall risk. |
| Conventional | Low calcium intake | Limits mineral deposition in new bone. |
| Immune‑related | High‑fat Western diet | Triggers adipose‑derived cytokines (TNF‑α) that boost RANKL. |
| Conventional | Physical inactivity | Decreases mechanical loading, lowering osteoblast signaling. |
Addressing the immune‑related side means tackling inflammation head‑on: adopt an anti‑oxidant rich diet (berries, leafy greens), limit processed sugars, and stay active.
Clinical Implications: Screening and Treatment
Doctors now consider inflammatory markers when evaluating bone health. A typical work‑up might include:
- DXA scan for BMD.
- Serum C‑reactive protein (CRP) to gauge systemic inflammation.
- Vitamin D 25‑OH level.
- Hormone profile (especially estradiol in post‑menopausal women).
If inflammation is a major contributor, treatment plans often blend anti‑osteoporotic meds with anti‑inflammatory strategies:
- Bisphosphonates - inhibit osteoclasts directly.
- Denosumab - blocks RANKL, curbing the immune‑driven resorption loop.
- Selective estrogen receptor modulators (SERMs) - mimic estrogen’s bone benefits without full hormonal exposure.
- Targeted anti‑cytokine therapy - TNF inhibitors used in rheumatoid arthritis also show modest bone‑preserving effects.
- Lifestyle prescription - anti‑inflammatory diet, weight‑bearing exercise, smoking cessation.
Research in 2024 showed that combining denosumab with a Mediterranean‑style diet reduced fracture incidence by 22% compared to denosumab alone.
Practical Checklist for Readers
- Get a baseline DXA scan if you’re over 50 or have a family history of fractures.
- Ask your clinician to test CRP and vitaminD levels.
- Include at least two servings of oily fish weekly - omega‑3s lower IL‑6.
- Aim for 150 minutes of moderate‑intensity weight‑bearing activity (walking, jogging, resistance training) each week.
- Limit processed snacks and sugary drinks - they spike TNF‑α.
- If you have an autoimmune condition, discuss bone‑protective medications with your rheumatologist.
Frequently Asked Questions
Can inflammation cause osteoporosis on its own?
Yes. Chronic inflammation raises cytokines that stimulate RANKL, which accelerates bone resorption even in people with normal calcium intake and activity levels.
Is there a blood test for bone loss related to the immune system?
While no single test diagnoses immune‑driven osteoporosis, doctors often check CRP, ESR, and vitaminD together with a DXA scan to get the full picture.
Do anti‑TNF drugs protect my bones?
Patients with rheumatoid arthritis who receive TNF inhibitors typically show slower loss of BMD compared with those on standard therapy, likely because the drugs reduce the inflammatory drive on RANKL.
How much vitaminD should I take to support both immunity and bone health?
Most adults benefit from 800‑1000IU daily, but individuals with low baseline levels or limited sun exposure may need 2000‑4000IU after a blood test confirms deficiency.
Are there specific exercises that counteract inflammation‑driven bone loss?
Weight‑bearing and resistance activities (e.g., squats, lunges, brisk walking) not only stimulate osteoblasts but also lower systemic CRP when done consistently for 12 weeks or more.





11 Comments
One cannot simply skim the surface of osteoimmunology without acknowledging the sublime intricacies that interlace cytokine cascades with skeletal fortitude; the mere mention of RANKL evokes a theatrical ballet of cellular actors, each vying for dominance upon the fragile stage of bone matrix.
The discourse presented herein traverses the formidable terrain of osteoimmunology with a gravitas befitting a treatise of antiquarian merit.
It meticulously delineates the symbiotic liaison between pro‑inflammatory cytokines and the RANK/RANKL/OPG axis, thereby elucidating the mechanistic conduit through which chronic inflammation begets skeletal fragility.
In particular, the exposition of estrogen’s modulatory capacity upon interleukin‑1 and tumor necrosis factor‑α is rendered with an erudition that commands admiration.
Such hormonal interplay, when contextualized within the post‑menopausal milieu, incontrovertibly rationalizes the precipitous surge in osteoporotic incidence among the female populace.
Equally noteworthy is the exposition of vitamin D as a dual‑purpose agent, orchestrating calcium homeostasis whilst tempering immunologic vigor.
The author’s inclusion of quantitative risk stratification, replete with a calculable algorithmic interface, bestows upon the lay reader an instrument of palpable utility.
Moreover, the tabular juxtaposition of immune‑related and conventional risk factors furnishes a comparative tableau that is both pedagogically sound and aesthetically pleasing.
From a clinical perspective, the recommendation to amalgamate denosumab therapy with a Mediterranean dietary regimen stands as a testament to the evolving paradigm of multimodal intervention.
The ancillary discussion of anti‑TNF agents reveals an astute appreciation for the pleiotropic benefits conferred upon bone mineral density beyond mere arthritic remission.
Furthermore, the treatise underscores the imperative of periodic DXA scanning, a diagnostic cornerstone whose prognostic significance cannot be overstated.
The narrative’s cadence, while at times reminiscent of academic prose, remains accessible due to judicious deployment of lay terminology interspersed amidst scientific nomenclature.
It is within this interplay of sophistication and approachability that the manuscript achieves its didactic pinnacle.
The author’s exhortation to engage in weight‑bearing exercise for a minimum of 150 minutes per week aligns harmoniously with extant guidelines promulgated by orthopedic societies.
The elucidation of omega‑3 fatty acids as modulators of interleukin‑6 further accentuates the article’s comprehensive scope.
In summation, the document succeeds in bridging the chasm between immunological insight and osteologic practice, thereby enriching the reader’s comprehension of a complex, interdependent system.
Such a synthesis, rendered with eloquence and empirical rigor, undeniably elevates the discourse surrounding osteoporosis to a plane of heightened scholarly respect.
Hey folks, great breakdown on how inflammation sneaks into bone loss. I appreciate the clear connection between cytokines like IL‑6 and the RANKL pathway – it really ties the immune side to bone remodeling. The practical checklist is super helpful, especially the suggestion to get a baseline DXA after 50. Adding a couple of omega‑3 rich meals each week is a simple tweak that can make a big difference. Keep the science accessible like this, it helps everyone stay informed.
Solid overview! If anyone’s managing a chronic condition, pairing your meds with weight‑bearing workouts can really tip the balance toward bone building. Remember, consistency beats intensity – a daily walk or light resistance routine keeps osteoblasts active. Also, don’t overlook vitamin D testing; getting those levels right supports both immunity and bone health. Stay proactive and consult your healthcare provider about a personalized plan. You’ve got this!
The article captures many key pathways, yet it glosses over the role of gut microbiota in modulating systemic inflammation and, by extension, bone turnover. A more thorough discussion on probiotics could enrich the therapeutic landscape. Additionally, the risk calculator omits lifestyle factors like smoking, which are pivotal in osteo‑immune dynamics. While the content is solid, integrating these nuances would elevate its comprehensiveness.
awesome summary of osteoimmunology its clear how cytokines drive bone loss especially with chronic diseases like RA the link between RANKL and osteoclast activation is spot on also vitamin D benefits both immunity and bone health so getting enough sunshine and supplements is key keep up the good work
Interesting read.
Great deep‑dive! 🦴🔬 The synergy between NF‑κB signaling and osteoclastogenesis is a classic example of cytokine‑mediated bone catabolism. Leveraging anti‑RANKL therapeutics like denosumab can modulate this axis effectively. Also, remember that CRP is a handy biomarker for systemic inflammation – good to track alongside BMD. Keep the data‑driven approach coming! 👍
Honestly this whole osteoimmunology hype feels like a fad from the EU. In proper British studies we’ve seen that diet and sunlight, not fancy cytokine talk, are the real bone saviours. Stop overcomplicatin it with RANKL drama – just get out and walk the streets, mate.
The piece is generally well‑written, however there are a few grammatical oversights worth noting. For instance, the sentence “The resultArea classList.remove” incorrectly mixes camelCase with a space; it should read “resultArea.classList.remove”. Additionally, “Vitamin D Level (ng/mL)” should consistently use the unit abbreviation without a backslash. Lastly, the phrase “weight‑bearing exercise (walking, jogging, resistance training) each week” would read more smoothly as “weight‑bearing exercise-such as walking, jogging, or resistance training-each week”. Attending to these details will enhance the article’s professionalism.
Well said, Albert. I’ll double‑check the code snippets and unit formatting for consistency. Accuracy in those technical details does matter for the reader’s trust.